
Androgenetic alopecia is the most common cause of hair loss in both men and women. It is a progressive condition that develops over years, gradually affecting appearance, self-confidence, and quality of life.
For this reason, every new treatment presented as “innovative” naturally attracts significant attention.
Clascoterone (Breezula®) is one of the most discussed recent developments, mainly because it promises local anti-androgenic action without systemic hormonal exposure. But what can patients realistically expect from it? Who benefits the most? And most importantly, can it replace a hair transplant?
This article provides a clear, medically grounded explanation of clascoterone—separating clinical reality from marketing claims.
Clascoterone is a topical androgen receptor antagonist.
Unlike systemic treatments, it does not reduce hormone production throughout the body. Instead, it acts locally at the level of the hair follicle, where androgenetic hair loss actually occurs.
✔ localized mechanism of action
✔ no meaningful systemic hormonal suppression
✔ designed as a long-term maintenance therapy
Its goal is not to regrow permanently lost hair, but to slow the progression of androgenetic alopecia by protecting follicles that are still active.
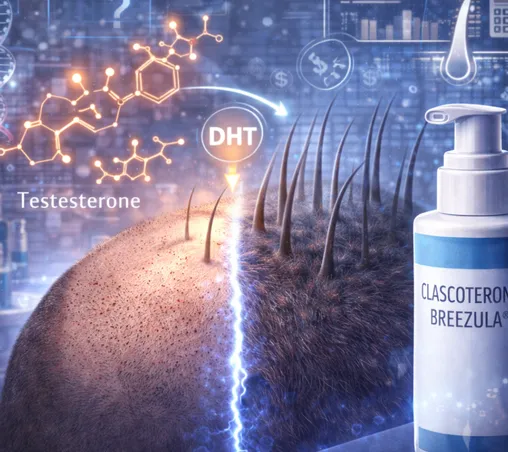
Androgenetic alopecia is driven by a genetically determined sensitivity of hair follicles to androgens, particularly dihydrotestosterone (DHT).
✔ testosterone is converted into DHT by the enzyme 5-alpha-reductase
✔ DHT binds to androgen receptors in the follicle
✔ follicles gradually miniaturize
✔ the growth phase (anagen) shortens
✔ hairs become thinner and shorter
✔ eventually, visible hair production stops
Once a follicle is irreversibly destroyed, no medication can recreate it. This biological fact is fundamental to understanding realistic treatment outcomes.
Clascoterone works by blocking androgen receptors at the follicular level, preventing DHT from exerting its damaging local effect.
✔ reduce local androgenic signaling
✔ slow follicular miniaturization
✔ preserve existing hair
✔ avoid systemic side effects
It is therefore a preventive and stabilizing treatment, not a regenerative one.
Originally developed and approved for acne, clascoterone 1% validated the concept of local anti-androgen therapy. Its use for hair loss remains off-label.
✔ proven dermatological safety
✔ off-label use in hair loss
This higher concentration was specifically developed for androgenetic alopecia and evaluated in Phase II and III clinical trials.
✔ formulation optimized for scalp penetration
✔ targeted follicular action
✔ minimal systemic exposure
Available clinical data demonstrate:
✔ a modest increase in hair density in treated areas
✔ a significant slowdown in disease progression compared to placebo
✔ improved stability of the hair growth cycle
However, one point must be clearly understood:
✔ clascoterone does not create new hair follicles
✔ observed improvements reflect preservation of existing hair
✔ it is not a hair regeneration treatment
Clascoterone is particularly useful in the following situations:
✔ early to moderate androgenetic alopecia
✔ diffuse thinning (especially vertex or mid-scalp)
✔ presence of viable follicles
✔ patients wishing to avoid systemic anti-androgens
This is a critical point often omitted from promotional content.
Clascoterone has limited or no effect in cases of:
✔ advanced baldness with completely smooth scalp areas
✔ long-standing, stable hair loss
✔ expectations of regrowth in fully bald zones
In these cases, hair transplantation remains the only restorative solution.
For a detailed explanation, visit our page on hair transplant.
Finasteride works by reducing systemic DHT production.
Clascoterone blocks DHT action locally at the follicle.
✔ finasteride: stronger effect, systemic exposure
✔ clascoterone: targeted action, minimal systemic impact
In modern clinical practice, this is not always an either-or decision, but rather an individualized treatment strategy.

Hair transplantation is the only permanent method for restoring areas where follicles have been destroyed.
However, it does not stop the androgenetic process affecting non-transplanted hair.
After a transplant, clascoterone can:
✔ protect native hair surrounding grafts
✔ slow ongoing miniaturization
✔ help maintain long-term aesthetic harmony
It has no negative effect on graft survival or growth.
Learn more about our surgical techniques: FUE hair transplant and DHI hair transplant.
Clascoterone works only while it is used.
✔ its stabilizing effect gradually diminishes after discontinuation
✔ androgenetic hair loss may resume
✔ transplanted hair remains unaffected
Like all maintenance therapies, consistency determines effectiveness.
The most durable results are achieved through a comprehensive approach combining:
✔ appropriate topical or oral treatments
✔ PRP therapy
✔ medical-grade scalp care
✔ lifestyle and nutritional optimization
This integrated strategy reflects current best practices in hair restoration medicine.
Thanks to its localized action, clascoterone offers:
✔ good tolerability
✔ high patient adherence
✔ minimal to no systemic side effects
This makes it suitable for long-term use, which is essential in a chronic condition such as androgenetic alopecia.
✔ hair transplantation restores what is lost
✔ clascoterone slows what continues to progress
✔ the most natural and durable outcomes rely on a well-planned combined strategy
You can explore real patient results in our before and after hair transplant gallery.
No. Clascoterone does not create new hair follicles. Its role is to preserve existing hair and slow follicular miniaturization in androgenetic alopecia.
Yes, in early to moderate stages. It helps slow progression and stabilize hair loss but does not reverse advanced baldness.
Its stabilizing effect gradually decreases after discontinuation. Androgenetic hair loss may resume over time, while transplanted hair remains unaffected.
They work differently. Finasteride acts systemically, while clascoterone works locally. The best option depends on the patient’s profile and long-term plan.
Clascoterone is generally well tolerated. Because it acts locally, systemic side effects are minimal to none.
Yes. Hair transplantation restores lost follicles but does not stop androgenetic hair loss in non-transplanted native hair.
In many cases, yes. Medical treatments help protect existing hair and maintain long-term aesthetic balance after surgery.
No. Medications preserve hair but cannot restore areas where follicles are permanently lost.
Native hair continues to follow its genetic pattern and may thin over time without maintenance therapy.
Transplanted hair is permanent, but overall density and harmony may change if native hair continues to thin.
Hair loss progression, donor capacity, and treatment needs vary from person to person.
The most accurate answers come from an individual medical evaluation, not general information.
• Free and confidential
• Reviewed by medical professionals
• Includes surgical and non-surgical options
No obligation. Medical evaluation only.
A correct plan from the beginning helps you:
• avoid unnecessary treatments
• protect your donor area
• achieve stable, long-term results
This content has been approved by Dr. Arslan Musbeh.
Your consultant is ready to answer your hair transplant questions, and you can also get a personalized online hair analysis.